

However, as the N protein sequence is much more stable than the spike, vaccines that include a component targeting the N protein are likely to be effective for longer. This could be similar to the current need for annual updating of influenza vaccines. There is some concern that if the spike sequence alters too much, then new vaccines will be required. Some changes to the sequence of SARS-CoV-2 have been reported over the course of this pandemic, with the most significant changes occurring in the spike protein. This means it's possible that a protective immune response against SARS-CoV-2 N protein could also offer some protection against other related coronaviruses, such as Mers.Īnother potential benefit that may arise from including N protein in SARS-CoV-2 vaccines is due to the low mutation rates seen in the N protein sequence.
This suggests that vaccines that induce N protein antibodies, as well as spike antibodies, could be valuable, as they would stimulate another way by which our immune response can eliminate SARS-CoV-2.Īdding N protein to SARS-CoV-2 vaccines could also be useful because N protein is very similar between different coronaviruses-much more so than the spike protein. We expect that this newly identified role for N protein antibodies in protecting against virus infection is important for SARS-CoV-2, and work is ongoing to explore this further. T cells recognize these fragments, identify cells as infected, then kill the cell and consequently any virus. Tiny fragments of N protein are then displayed on the surface of infected cells. We have shown that N protein antibodies that get inside cells are recognized by TRIM21, which then shreds the associated N protein. Whereas antibodies are typically thought to only work outside of cells, TRIM21 is only found inside cells. We have studied another virus containing an N protein called lymphocytic choriomeningitis virus and shown a surprising role for an unusual antibody receptor called TRIM21. Our latest work from the MRC Laboratory of Molecular Biology in Cambridge has revealed a new mechanism for how N protein antibodies can protect against viral disease. Therefore, N protein antibodies cannot block virus entry, will not be measured in neutralization assays that test for this in the lab, and so have largely been overlooked. This is because N protein is only found inside the virus particle, wrapped around the RNA. But how N protein antibodies protect us from infection has been a long-standing mystery.
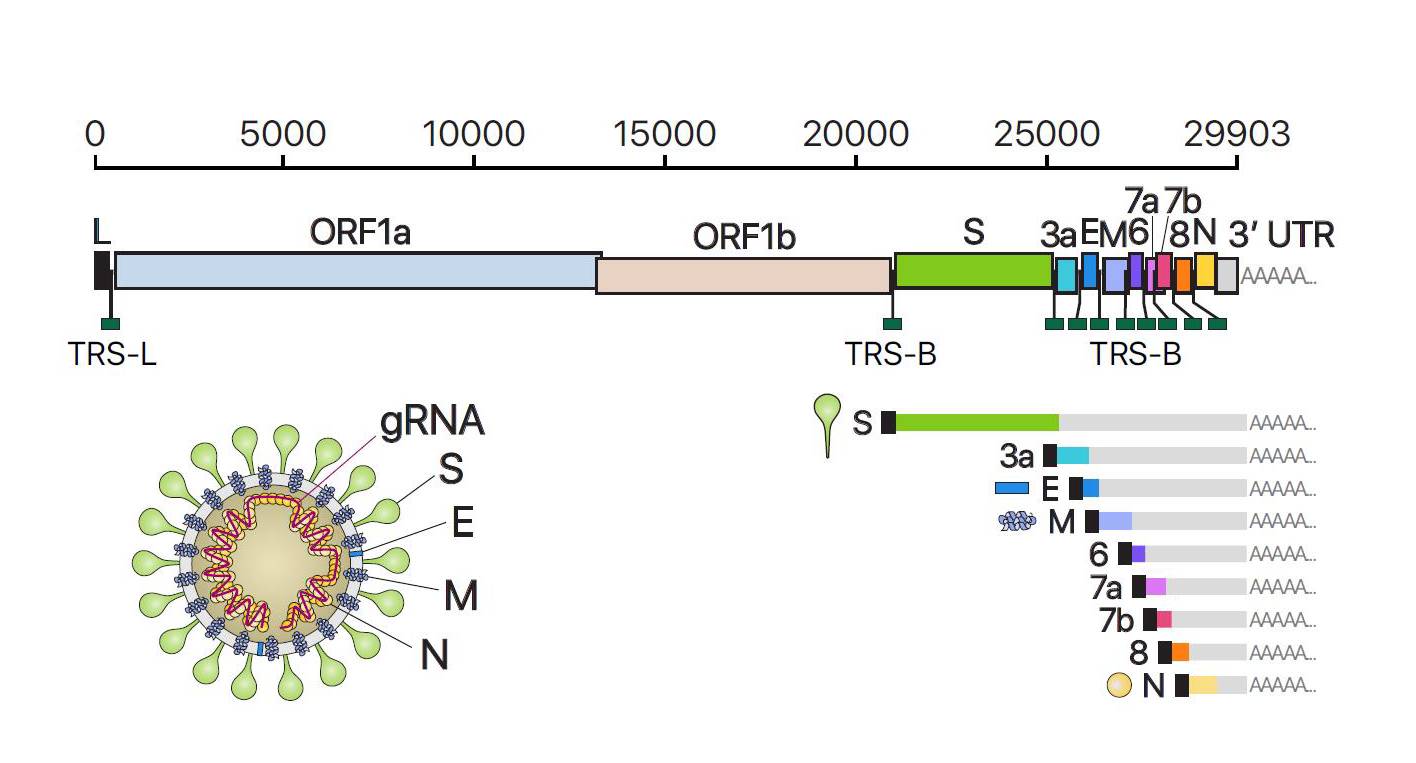
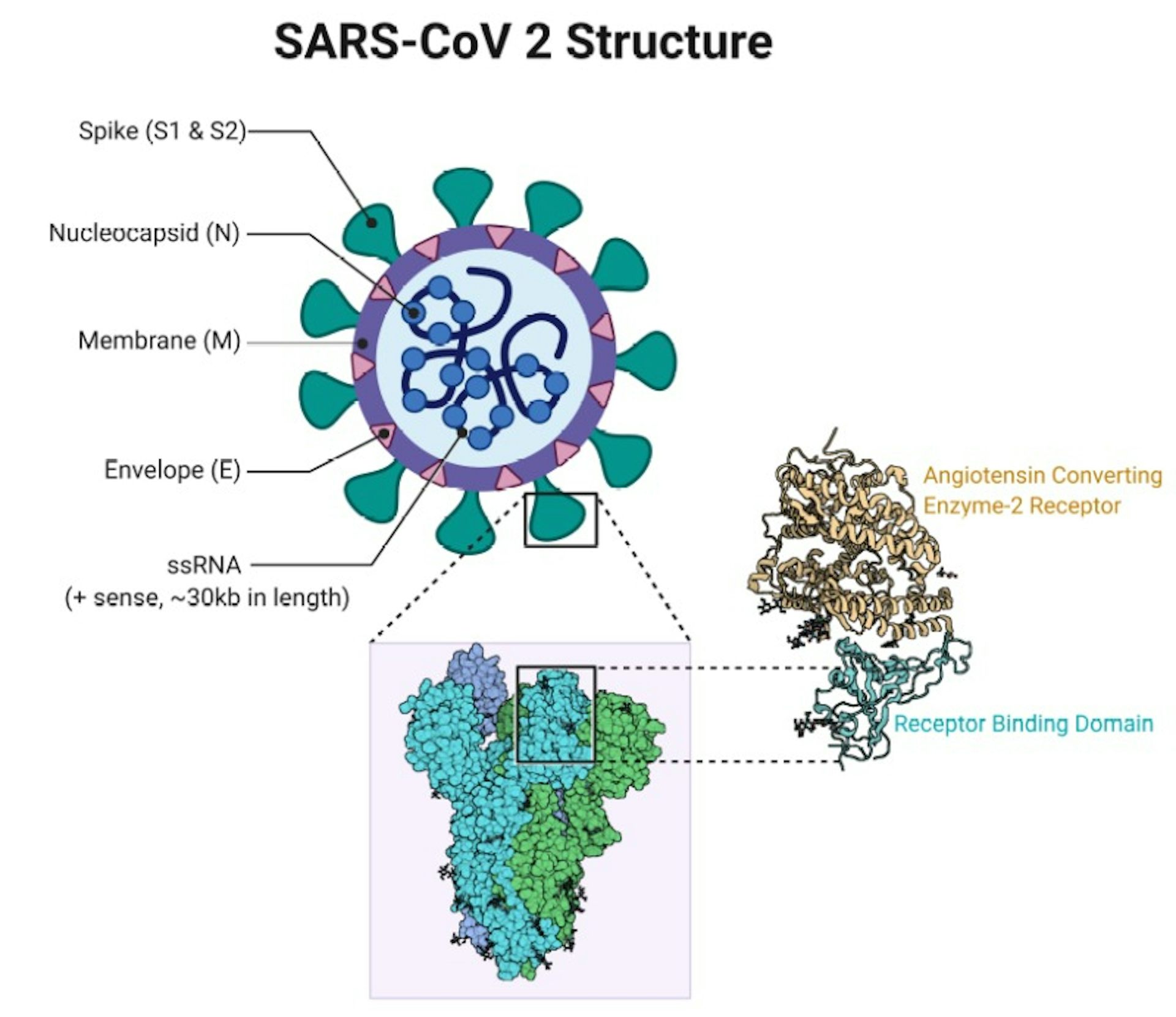
This is the same for many different viruses that also have N proteins. So how important are immune responses to these different proteins, and does it matter that the first vaccines will not replicate these?įollowing SARS-CoV-2 infection, researchers have discovered that we actually make the most antibodies to the N protein – not the spike protein. In a natural infection, our immune system recognizes all of these proteins to varying degrees. There are, in fact, four different proteins that form the overall structure of the virus particle: spike, envelope (E), membrane (M) and nucleocapsid (N). However, the SARS-CoV-2 virus is more complicated than just a spike protein. When our own cells make the spike protein, our immune response will recognize it as foreign and start making antibodies and T cells that specifically target it.

The other two vaccines deliver the spike protein gene directly as mRNA wrapped in a nanoparticle. The Oxford vaccine achieves this by introducing the spike protein gene via a harmless adenovirus vector. The three most advanced vaccines (from Oxford/AstraZeneca, Pfizer/BioNTech and Moderna) all work by getting our own cells to make copies of the virus spike protein. If a person has antibodies that can recognize the spike protein, this should stop the virus in its tracks. Virus replication only happens inside cells, so blocking entry prevents more virus being made. The spike protein is the focus of most COVID-19 vaccines as it is the part of the virus that enables it to enter our cells.


 0 kommentar(er)
0 kommentar(er)
